All Features

ISO
In today’s digital age, the question isn’t whether you’ll experience a cybersecurity attack, but when this might occur. Cybercriminals strike when you least expect it, with devastating consequences for your day-to-day operations. If your organization is lucky, it can block the attacker and limit…

Bennie Caldwell
In manufacturing, failure isn’t an option—it’s a liability. A defective part or a missed delivery triggers a chain reaction that can disrupt schedules, undermine trust, and drain resources.
So when someone suggests a strategy with the word fail in it, skepticism is understandable, because…

David Hall Rode
In 2025, there’s been a marked increase in FDA warning letters. During the second quarter of 2025 alone, the U.S. Food and Drug Administration (FDA) issued 172 warning letters. A notable enforcement surge occurred in September 2025 when the FDA released 80 warning letters in a single week. Although…

ETQ—Part of Hexagon
Even the smallest manufacturer would never consider using a typewriter to develop an invoice, or manage a sales prospect list from a Rolodex. So why, when it comes to quality management, are they often still using manual methods or home-brewed software that was never intended for today’s quality…

Artem Kroupenev
Quality has always been a defining metric in manufacturing when it comes to industry trust, brand longevity, and customer loyalty. Manufacturers are already expected to abide by stringent regulations. But as economic complexity rises and experienced operators retire, maintaining consistent quality…

Bryan Christiansen
From manufacturing and mining to hospitality and healthcare, computerized maintenance management systems (CMMS) have become all but essential. Wherever there are assets to maintain, a CMMS plays a critical role in reducing downtime, controlling costs, and keeping operations running smoothly.
But…

Mat Gilbert, John Robins
Physical AI—the embedding of digital intelligence into physical systems—is a promising but sometimes polarizing technology. Optimists point to the upside of combining AI and physical hardware: robot-assisted disaster zone evacuations, drone deliveries of critical supplies, and driver assistance…

ISO
In 2021, container ships idled for weeks outside the Port of Los Angeles, a stark visual reminder of just how fragile modern supply-chain reliability had become. The backlog sent shockwaves across industries. Factories stalled, shelves emptied, and businesses scrambled for alternatives. It was a…

Elizabeth Weddle
The quality systems most medtech teams are stuck with aren’t built for how they work today. 21 CFR Part 820 was authorized by the Federal Food, Drug, and Cosmetic Act of 1978, long before the software industry even existed. And while the regulations themselves aren’t going anywhere, the world they…
Adam Zewe
What can we learn about human intelligence by studying how machines “think?” Can we better understand ourselves if we better understand the artificial intelligence systems that are becoming a more significant part of our everyday lives?
These questions may be deeply philosophical, but for Phillip…

Stephanie Ojeda
Implementing a new quality management system (QMS) is no small task, especially for life science companies faced with stringent regulatory requirements and a high validation burden. Entrenched legacy systems compound the problem as organizational inertia and complacency lead companies to make do…

Brian Brooks
The arrival of artificial intelligence (AI) in quality management has been met with a mixture of hype and skepticism. Is it just a faster anomaly detector, or is it truly transformative?
The answer depends on how we frame the problem. If we see AI merely as a way to speed up quality processes we…

CANEA
The role of quality leaders, and quality itself, is expanding. It includes thinking strategically, solving problems, implementing improvements, and driving change throughout the organization.
Quality leadership also requires managing challenges and anticipating what lies ahead regarding quality…

Stephanie Ojeda
When organizations implement an enterprise quality management system (EQMS), the instinct is often to begin with high-visibility processes like corrective and preventive action (CAPA) or supplier quality. While these functions are critical, starting there can be a misstep. Without the right…

William A. Levinson
My June 2025 article, “How to Avoid FDA Warning Letters,” points out that inadequate corrective and preventive action (CAPA) is a major reason for warning letters, and also introduces the role of failure mode effects analysis (FMEA) in preventing trouble in the first place. The U.S. Food and Drug…

Mike Figliuolo
I had a great conversation with a friend of mine. He was bemoaning the fact that his company was almost completely dependent on one huge customer. He saw the inherent risks in that relationship but confessed that his organization had a bad habit it couldn’t kick. It had succumbed to the addiction…

Lexi Sharkov
We’d be willing to bet your key collaborators aren’t all in the same building. Your team members, contract partners, clients, and suppliers are likely scattered across the globe. That makes collecting physical, “wet ink” signatures nearly impossible and turns digital approvals into a daily…

MasterControl Inc.
Ninety days to implementation vs. 12 to 18 months with traditional systems: That’s not just an incremental improvement—it’s a complete reimagining of what’s possible in life sciences quality management.
In the highly regulated life sciences industry, quality management system (QMS) implementations…

Lexi Sharkov
When an issue arises, it’s important to take quick action. Whether that means launching a software patch, pulling a batch, or halting the use of a reagent, it’s critical to tackle the immediate problem.
But just as critical as “How do we fix this?” is “How do we make sure this doesn’t happen again…

Quality Digest, Jason Chester
Today, manufacturing is largely shaped by supply chain volatility, complex labor dynamics, and—like most global industries—the rise of AI. Adopting AI technologies on the shop floor can help manufacturers minimize operational costs, mitigate risk, and optimize processes, which drives efficiency and…
Oak Ridge National Laboratory
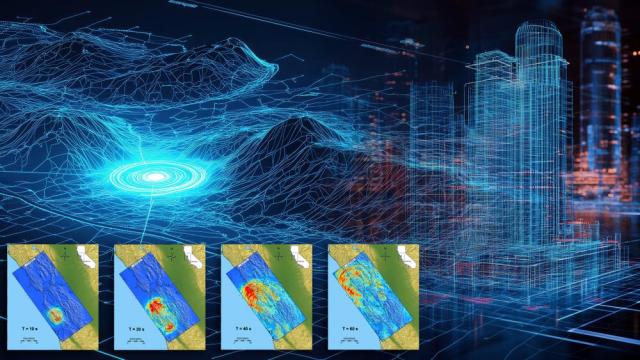
Simulations still can’t predict precisely when an earthquake will happen. Still, with the incredible processing power of modern exascale supercomputers, they can now predict how they will happen and how much damage they will likely cause.
Imagine a colossal earthquake strikes the California coast…

Walter Nowocin
Software selection, implementation, and ongoing maintenance are critical stages in the life cycle of biomedical software systems such as asset and calibration management platforms. Yet few industry resources provide detailed, practical guidance for managing these processes effectively.
One notable…

Judy Fainor
What if your quality system could detect and initiate corrective actions for equipment deviations before they affect product quality?
It’s a compelling vision—and one that’s becoming increasingly achievable through AI-enabled automation. But let’s be clear: We’re not there yet. What we do have is…

Mike Regan
In July 2024, CrowdStrike rolled out a software update that crashed more than 8 million Windows systems worldwide. The faulty release disrupted hospitals, grounded flights, halted banking operations, and affected government services. Comparable to a major cyberattack, the incident caused more than…

William A. Levinson
A vital concept from the chemical process industry, management of change (MOC) relates primarily to safety. It means that whenever we change a factor in a cause-and-effect diagram (e.g., machine, material, manpower, method, measurement, environment, or any other factor), we create risks of…